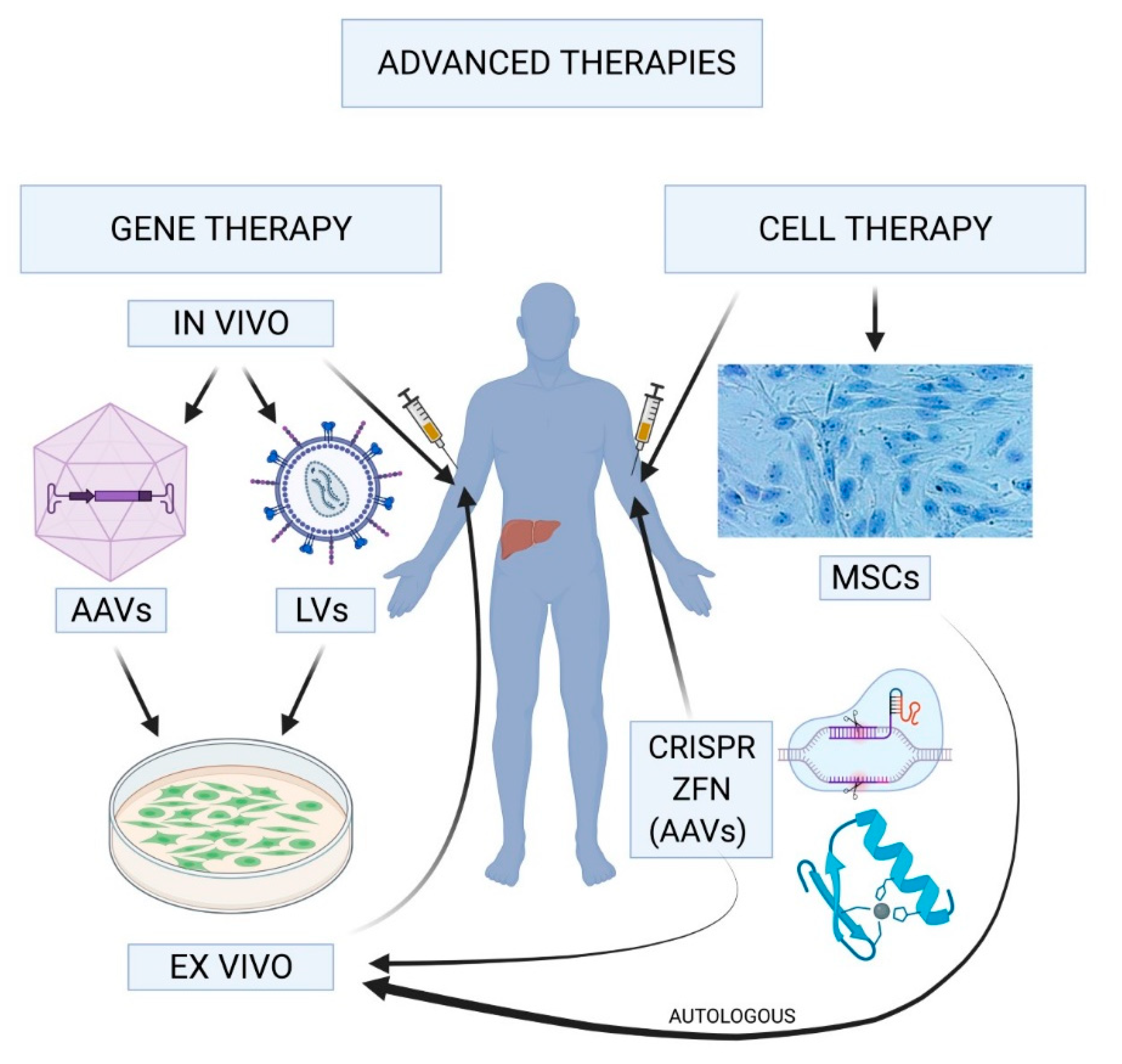
Gene therapy for hemophilia is emerging as a groundbreaking treatment, offering patients a chance to live free from the constant threat of bleeding episodes. This revolutionary approach utilizes innovative techniques to deliver genes that can produce the missing clotting factor, fundamentally changing how hemophilia is managed. With advancements such as Hemgenix, approved by the FDA, the landscape of hemophilia treatment is evolving, potentially eliminating the need for traditional clotting factor therapy. As researchers unveil new gene therapy breakthroughs, patients are beginning to see the light at the end of the tunnel, where daily injections may no longer dictate their lives. By harnessing the power of genetic science, gene therapy is paving the way for a future where individuals with hemophilia can experience more freedom and a better quality of life.
When it comes to managing hemophilia, innovative therapies are making waves in the medical field, especially through advanced genetic interventions. Known as genetic modification strategies, these treatments offer a transformative solution by addressing the root causes of the disorder, allowing the body to regain its ability to produce essential proteins that aid in blood clotting. With the arrival of treatments like Hemgenix, patients are finding hope in alternatives to traditional clotting therapies. This shift signifies a monumental leap in hemophilia management, as gene therapy not only aims for symptom relief but also seeks to correct the underlying genetic flaws. As the field progresses, ongoing studies and clinical trials are vital to ensuring the efficacy and safety of these new therapeutic options for those living with hemophilia.
Understanding Gene Therapy for Hemophilia
Gene therapy for hemophilia represents a groundbreaking approach in treating this genetic disorder. Hemophilia is caused by mutations in genes responsible for producing clotting factors essential for blood coagulation. By utilizing gene therapy, medical professionals can introduce healthy copies of these genes into a patient’s cells, significantly increasing the production of the required clotting factor. This strategy not only aims to reduce the frequency of bleeding episodes but also seeks to improve the overall quality of life for individuals living with hemophilia. As seen in the case of Terence Blue, who became the first patient in New England to receive Hemgenix, gene therapy can potentially transform the approach to managing hemophilia by offering patients long-term solutions instead of ongoing treatments.
This innovative therapy is designed to provide a lasting cure for hemophilia, distinguishing it from traditional treatments like clotting factor therapy that require regular administration. By addressing the root cause of the disease, gene therapy represents a significant advancement in hemophilia treatment. The success of Hemgenix has generated immense excitement in the medical community, as it not only provides hope for individuals like Blue but also opens new avenues for research into other genetic disorders. The breakthrough signifies a paradigm shift in the development of therapies aimed at genetic diseases, aligning with the growing movement towards precision medicine in healthcare.
The Evolution of Hemophilia Treatment Options
Over the years, hemophilia treatment has evolved from rudimentary blood transfusions to advanced clotting factor therapies, reflecting significant progress in medical science. Initially, patients relied on treatments derived from pooled plasma, which posed significant health risks such as the transmission of viruses like HIV. With the introduction of recombinant clotting factors in the 1990s, the safety of hemophilia treatment improved dramatically. These modern therapies enabled patients to maintain their daily lives with less concern over unexpected bleeding incidents. However, despite these advancements, many individuals still face challenges associated with frequent injections and managing their condition over their lifetime.
The emergence of gene therapy adds an exciting new layer to these treatment options. Unlike traditional clotting factor therapies, which require consistent administration, gene therapy provides a long-term solution potentially allowing patients to live without daily injections. With products like Hemgenix gaining FDA approval, discussions around the future of hemophilia treatment are optimistic. Nevertheless, the integration of gene therapy into the healthcare system also raises questions about accessibility, pricing, and patient education, which need to be addressed to ensure that these innovative solutions can benefit all individuals affected by hemophilia.
Market Challenges in Gene Therapies
While gene therapy presents unprecedented opportunities for hemophilia treatment, it also faces significant market challenges. The complexities of pricing and insurance coverage can create barriers to access, particularly for expensive therapies like Hemgenix, which costs upwards of $3.5 million. These high price tags stem from the intricate research and development processes involved in creating such therapies. As pharmaceutical companies develop these treatments, they must balance the costs of development with market demand. If the treatment is priced too high, it may dissuade patients and healthcare providers from embracing these cutting-edge solutions.
The situation is compounded by the dynamic nature of the healthcare market, where patient acceptance and insurance reimbursements dictate the viability of new therapies. As seen in recent examples, some therapeutic agents have been withdrawn from the market due to insufficient uptake or reimbursement issues. This highlights the need for a comprehensive strategy that includes patient education about the benefits and risks of new gene therapies, as well as advocacy for fair pricing models that consider the unique nature of gene therapy as a potentially one-time treatment.
The Revolutionary Role of Hemgenix in Hemophilia Therapy
Hemgenix has become a focal point in the discussion surrounding gene therapy for hemophilia B. Developed by CSL Behring and receiving FDA approval in November 2022, this innovative treatment has demonstrated promising results in clinical trials, with the potential to eliminate the need for regular clotting factor injections. By targeting patients with hemophilia B, Hemgenix essentially corrects the underlying genetic mutation causing the deficiency in factor IX, allowing the body to produce its own clotting factor. This represents a significant shift from conventional therapies that require continuous management and regular hospital visits for injections.
The excitement surrounding Hemgenix is indicative of the broader trends in gene therapy aimed at genetic disorders. As researchers refine vector technologies, which are essential for the effective delivery of gene therapies, the potential to treat not only hemophilia but various other genetic conditions becomes increasingly feasible. With ongoing research and the success of treatments like Hemgenix, there is hope that gene therapy can lead to a future where conditions once thought incurable can be effectively managed or even eradicated.
Future Implications of Gene Therapy for Hemophilia Patients
The advent of gene therapy for hemophilia patients heralds a new era in treatment that promises to reduce the burden of chronic management of the condition. As more patients like Terence Blue experience the life-changing effects of therapies such as Hemgenix, there is an increasing call for broader accessibility and integration of these therapies into standard care practices. The implications are vast: not only do patients stand to gain improved health outcomes, but the overall healthcare system may benefit from reduced long-term costs associated with frequent treatment and emergency care.
Moreover, ongoing advancements in gene therapy are likely to inspire further research into tailored treatment options for other genetic disorders. As the field continues to evolve, there is optimism that the successes achieved in hemophilia will pave the way for similar breakthroughs in other conditions, enhancing the lives of many more patients who currently have limited treatment options. The intersection of innovation, policy-making, and patient care will be crucial in shaping the future landscape of hemophilia and other genetic therapies.
Clotting Factor Therapy: A Historical Perspective
Clotting factor therapy has long been the cornerstone of hemophilia treatment, evolving significantly since its inception. Historically, patients relied on fresh blood products or pooled plasma, which although effective, carried significant risks of blood-borne infections. The 1990s marked a turning point with the development of recombinant clotting factors, which significantly increased the safety profile of treatments. These advancements allowed for more precise dosing and extended intervals between treatments, substantially improving the quality of life for people with hemophilia.
Despite these advances, clotting factor therapy has its limitations. Patients still face challenges related to the need for ongoing treatments and the associated healthcare costs. Many remain hopeful that as gene therapy advances, these therapies might either supplement or even replace traditional clotting factor therapy. Innovations such as Hemgenix offer a glimpse into a future where individuals with hemophilia may no longer need to rely on frequent injections or live in fear of spontaneous bleeding.
Real-World Experiences of Patients Undergoing Gene Therapy
The real-world experiences of patients like Terence Blue underscore the transformative impact of gene therapy for hemophilia. For years, Blue endured the psychological and physical toll of managing his condition, from regular hospital visits to the anxiety associated with spontaneous bleeds. With the advent of Hemgenix, many patients are finding renewed hope as they transition from a lifetime of maintaining their condition to potentially achieving normal clotting function with a single treatment. Stories like Blue’s not only highlight the medical breakthroughs but also the emotional journey many patients undergo during this transformative time.
Additionally, patient testimonials and data from clinical trials are crucial in understanding the impacts of gene therapy. As patients report improved outcomes such as increased levels of clotting factor and reduced bleeding episodes, it paints a hopeful picture for future patients. However, it’s vital to continue monitoring these patients’ long-term responses, ensuring that the benefits of gene therapy are sustained over time, which will ultimately shape the path toward optimizing treatment strategies for hemophilia.
Navigating the Challenges of Patient Awareness and Education
One of the prevailing challenges in the acceptance of gene therapy for hemophilia is the level of patient awareness and education regarding these new treatments. Although innovative therapies like Hemgenix offer significant benefits, many patients may be hesitant to embrace new treatment modalities due to concerns about long-term efficacy, potential side effects, and costs. Therefore, healthcare providers play a critical role in delivering accurate information that addresses these concerns, helping patients understand the transformative potential of gene therapy.
Educational initiatives are essential to ensure patients are informed about their options. This includes providing insights into the benefits and risks of gene therapy, as well as practical information about treatment access and financial aspects. As awareness of gene therapy grows, it is likely that more patients will consider this opportunity, contributing to better health outcomes and improved quality of life for those managing hemophilia.
Research Advancements in Gene and Cell Therapies
Research into gene and cell therapies has seen unprecedented growth, with a significant focus on conditions such as hemophilia. The breakthroughs achieved through ongoing studies not only enhance our understanding of gene therapy mechanisms but also refine delivery methods and improve patient outcomes. In the past few years, the approval of therapies like Hemgenix demonstrates the rapid pace at which research is translating into practical applications, moving from concept to clinic faster than ever before.
With a growing catalog of gene therapies, researchers are hopeful about the prospects of future innovations that could treat a wider range of genetic disorders. The synergy between laboratory research, clinical trials, and real-world applications signifies a promising future not just for hemophilia but for a host of other conditions as well. By fostering collaboration among researchers, clinicians, and patients, the aim is to bring forth effective gene therapies that can redefine the landscape of treatment.
Frequently Asked Questions
What advancements has gene therapy for hemophilia brought to hemophilia treatment?
Gene therapy for hemophilia represents a groundbreaking advancement in hemophilia treatment, particularly with therapies like Hemgenix. This innovative approach aims to correct the genetic mutations causing hemophilia, potentially alleviating the need for routine clotting factor therapy. By directly introducing functional copies of the defective gene into the patient’s liver cells, gene therapy can enable the body to produce the clotting factor naturally, significantly improving patients’ quality of life and reducing dependence on frequent infusions.
How does Hemgenix work as a treatment for hemophilia B?
Hemgenix is a gene therapy specifically designed for treating hemophilia B. It utilizes a modified virus to deliver a corrected copy of the gene responsible for producing clotting factor IX directly into the liver. This therapy prompts the liver to start generating its own clotting factor, potentially transforming hemophilia management by decreasing or eliminating the need for regular clotting factor therapy.
What are the benefits of gene therapy breakthroughs like Hemgenix for hemophilia patients?
Gene therapy breakthroughs like Hemgenix offer numerous benefits for hemophilia patients, including reduced frequency of treatment, decreased reliance on clotting factor infusions, and the possibility of significantly improved bleeding control. Patients like Terence Blue have reported experiencing faster healing times and fewer spontaneous bleeds, representing a substantial shift in the quality of life for those living with hemophilia.
Are there any risks associated with gene therapy for hemophilia?
While gene therapy for hemophilia, including options like Hemgenix, holds significant promise, it is not without risks. Potential side effects can include liver enzyme elevation and immune responses to the therapy. Patients are closely monitored during and after treatment to manage any adverse effects. It is essential for patients to discuss potential risks and benefits with their healthcare providers before undergoing treatment.
How does the cost of gene therapy for hemophilia, like Hemgenix, affect patient access?
The cost of gene therapy for hemophilia, such as Hemgenix, can be prohibitively high, with listings around $3.5 million. While insurance negotiations can reduce this cost, high prices pose substantial accessibility issues for many patients. Market dynamics and the willingness of payers to cover such treatments can significantly impact how readily these therapies become available, potentially limiting access to those who need them most.
What impact does gene therapy have on the future of hemophilia treatment?
The advent of gene therapy for hemophilia, particularly through approaches like Hemgenix, dramatically alters the future landscape of hemophilia treatment. It introduces the potential for long-term, possibly curative solutions rather than managing symptoms through regular clotting factor therapy. As research continues and more therapies are developed, the hope is to provide effective treatment options that can permanently alleviate the burden of hemophilia.
What should patients consider before starting gene therapy for hemophilia?
Before starting gene therapy for hemophilia, patients should consider several factors, including the therapy’s potential benefits and risks, their personal medical history, and the financial implications of treatment. It’s crucial to have comprehensive discussions with healthcare providers about expectations, side effects, and the monitoring required post-treatment to ensure informed decision-making.
What results have been observed in clinical trials for hemophilia gene therapy?
Clinical trials for hemophilia gene therapy, including those for Hemgenix, have reported promising results. For instance, 94% of participants maintained sufficient factor IX levels without the need for prophylactic treatment three years post-therapy. These findings indicate that gene therapy may offer substantial long-term benefits, reducing bleeding episodes and improving overall health outcomes for hemophilia patients.
Can gene therapy for hemophilia be considered a cure?
While gene therapy for hemophilia, such as Hemgenix, is a major advancement and can lead to long-lasting effects, it is cautious to use the term ‘cure.’ Though many patients experience significantly improved clotting factor levels and reduced bleeding incidence, ongoing research is needed to fully understand the durability of the response and whether these treatments can provide a permanent solution.
| Key Points |
|---|
| Terence Blue became the first patient in New England to receive Hemgenix, a gene therapy for hemophilia B, at Brigham and Women’s Hospital. |
| Hemgenix was approved by the FDA in November 2022 and uses a virus to modify liver cells to produce clotting factor IX, which is deficient in hemophilia patients. |
| The therapy represents a significant advancement in hemophilia treatment, potentially reducing the need for regular infusions of clotting factor. |
| Despite the promise of gene therapy, it must contend with high costs and market realities, impacting patient access and adoption. |
| Terence Blue noticed a substantial improvement in his condition following the therapy, experiencing faster healing and reduced bleeding episodes. |
Summary
Gene therapy for hemophilia has emerged as a groundbreaking treatment, providing new hope for patients like Terence Blue. With the approval of Hemgenix, a gene therapy that aims to modify liver cells to produce necessary clotting factors, individuals with hemophilia B may soon enjoy a life less burdened by constant treatments and bleeding risks. This innovative approach not only marks a significant advancement in hemophilia management but also underscores the potential of gene therapy to transform lives permanently, paving the way for more efficient healthcare solutions in the future.