The recent advancements in TIM-3 Alzheimer’s treatment present a promising frontier in Alzheimer’s therapy that leverages the immune system’s inherent functions. Researchers have uncovered how the inhibition of TIM-3, an immune checkpoint molecule, empowers microglia to effectively combat the accumulation of amyloid plaques in the brain. With the ability to enhance microglial function and promote memory improvement, these findings signal a potential breakthrough in how we approach the treatment of Alzheimer’s disease. This novel strategy could redefine therapeutic paradigms, especially in the wake of previous challenges faced in Alzheimer’s trials. By repurposing techniques used for cancer treatments, TIM-3 Alzheimer’s treatment could unlock new pathways towards managing and potentially reversing cognitive decline associated with Alzheimer’s.
Innovative strategies targeting immune mechanisms could transform the landscape of Alzheimer’s care, particularly through therapies that inhibit specific immune checkpoint molecules like TIM-3. These approaches aim to restore the normal functioning of microglia, the brain’s immune cells, which play a crucial role in maintaining neurological health and clearing harmful plaques. By enhancing the ability of these cells to clear amyloid deposits, such treatments could significantly enhance cognitive function and improve overall memory retention. As the medical community explores novel tactics once reserved for cancer therapy, these discoveries could reshape our understanding of Alzheimer’s disease management. The intersection of Alzheimer’s treatment and immune therapy opens up exciting possibilities for future research and patient outcomes.
TIM-3: A Potential Breakthrough in Alzheimer’s Treatment
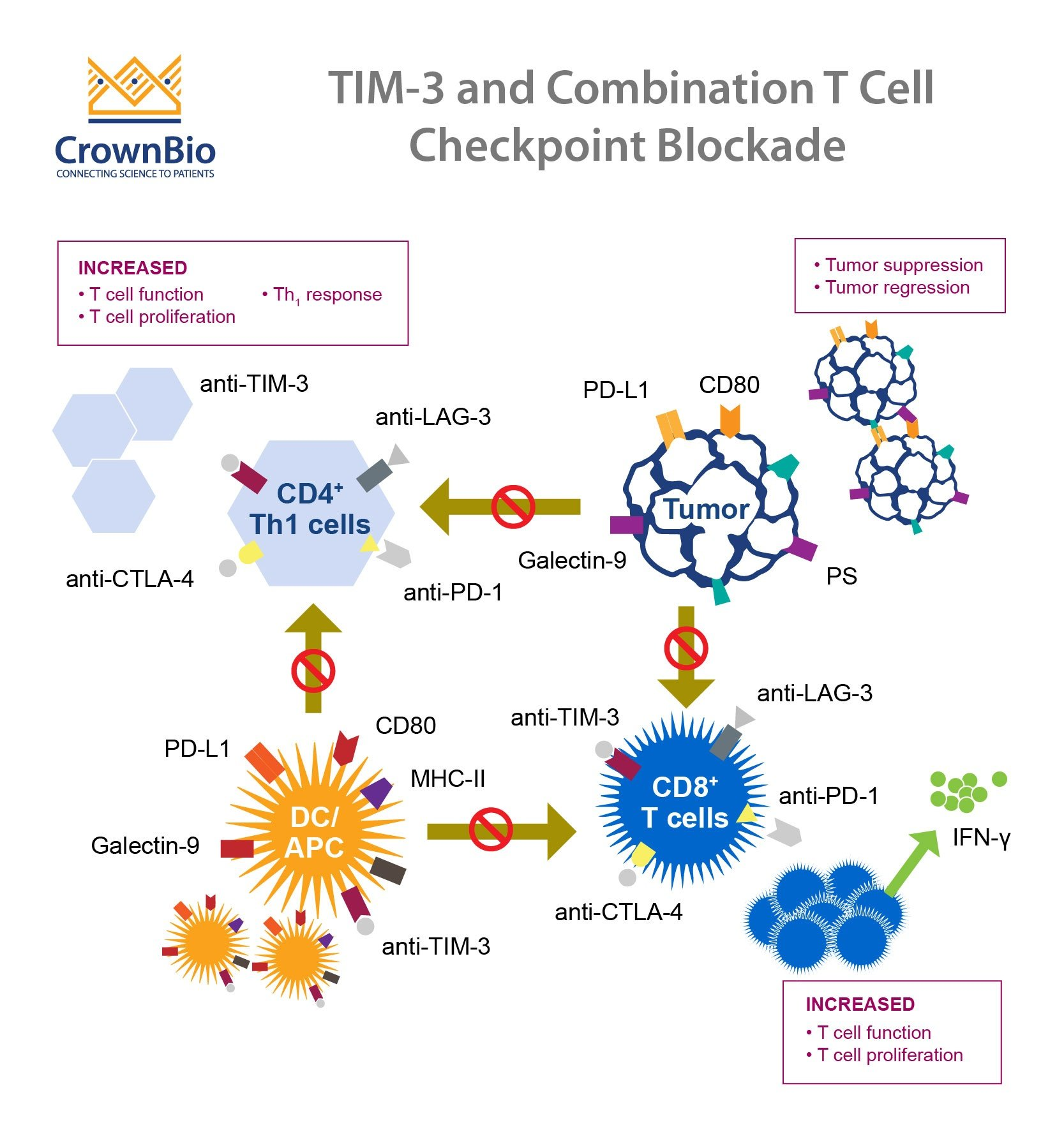
Recent findings showcase the potential of TIM-3 (T-cell immunoglobulin and mucin domain 3) as a revolutionary target for Alzheimer’s treatment. Researchers have discovered that deleting the gene responsible for TIM-3 expression in mouse models results in improved microglial activity, allowing these crucial immune cells to clear out amyloid plaques more efficiently. As a result, memory improvements were noted in these animals, illustrating TIM-3’s role not only as an immune checkpoint molecule but also as a hindrance in Alzheimer’s pathology. This highlights the importance of exploring TIM-3 in the context of Alzheimer’s therapy, offering new avenues for drug development that can enhance cognitive function and mitigate the disease’s effects.
Furthermore, TIM-3 is genetically linked to late-onset Alzheimer’s disease, affecting a significant majority of cases. The study reflects a shift in understanding how immune checkpoint molecules operate beyond their traditional roles in cancer treatment, paving the way for innovative therapeutic strategies. Identifying TIM-3’s function in microglial response could lead to powerful interventions aimed at rejuvenating cognitive processes in Alzheimer’s patients. This represents a paradigm shift in Alzheimer’s therapy, encouraging exploration of immune modulation as a viable approach for combating the disease.
Exploring Immune Checkpoint Molecules in Alzheimer’s Therapy
The application of immune checkpoint molecules in Alzheimer’s treatment taps into an exciting realm of neurology and immunology. Checkpoint molecules, including TIM-3, play a vital role in regulating immune responses. By manipulating these molecules, researchers aim to unleash the brain’s innate defense mechanisms against the toxic amyloid beta plaques that characterize Alzheimer’s. The underlying principle of this approach is to ‘turn off’ these checkpoints, thereby encouraging activated microglia to effectively clear plaque deposits, facilitating memory restoration and cognitive functions.
Understanding the dynamic interaction between the immune system and Alzheimer’s pathology opens new dialogues on potential therapies. By utilizing anti-TIM-3 antibodies or small inhibitors to diminish its inhibitory effects, emerging therapies could enhance the microglial response in ways larger drug trials have previously struggled to achieve. This exploration not only revisits established cancer treatment strategies but also creatively applies them to a neurodegenerative context, significantly shifting how researchers might approach Alzheimer’s therapy in future studies.
The Role of Microglia in Cognitive Health
Microglia, the immune cells of the brain, play a pivotal role in maintaining cognitive health and memory function. Under normal circumstances, they function to prune unnecessary synapses during development and clear debris from the brain. However, as Alzheimer’s disease progresses, microglial function deteriorates, primarily due to factors like the expression of TIM-3 which hinders their ability to clear amyloid plaques. This dysfunctional state contributes to disease progression by allowing toxic accumulations to persist and exacerbate cognitive decline.
The study of microglia’s role has significant implications for Alzheimer’s therapy. By understanding how microglial activity correlates with memory improvement, researchers can enhance therapeutic strategies that target these cells. Interventions that rejuvenate microglial function could have a profound impact on memory retention and cognitive health in Alzheimer’s patients, emphasizing the need for targeted approaches that restore microglial vitality and their ability to interact with amyloid beta effectively.
Memory Improvement through Immune Modulation
Recent studies indicate a clear link between immune modulation and memory improvement in Alzheimer’s disease. The deletion of TIM-3 in mouse models demonstrated that enhanced microglial activity could reverse cognitive deficits associated with plaque accumulation. This suggests that therapies aimed at modulating the immune response could lead to significant advancements in cognitive health, providing a glimmer of hope for those affected by Alzheimer’s.
In essence, targeting immune pathways, particularly through the modulation of molecules like TIM-3, could provide a novel strategy for improving memory. By unlocking the potential of microglia to clear neurotoxic debris, new therapeutic frameworks may emerge, promising to uplift the quality of life for Alzheimer’s patients. Aligning this approach with existing Alzheimer’s therapy paradigms may ultimately lead to innovative and effective treatments.
Lessons from Cancer Therapies for Alzheimer’s Strategies
The use of strategies developed for cancer treatment carries powerful implications for Alzheimer’s therapy, particularly those involving immune checkpoint molecules. As the research into TIM-3 demonstrates, the mechanisms by which tumors evade immune detection can be redirected to prompt a robust immune response against amyloid plaques. By borrowing techniques from oncology, researchers are beginning to tailor similar approaches to invigorate the immune system’s capacity to combat Alzheimer’s.
As academic studies continue to uncover the complexities of immune interactions in neurodegeneration, leveraging lessons learned from cancer therapies could result in the development of unique Alzheimer’s strategies. This cross-disciplinary approach not only provides hope for innovative treatments but also underlines the importance of looking at how existing therapies can be adapted to address other diseases, particularly those impacting the brain.
The Journey of Research: From Mice to Humans
The roadmap from animal models to human applications is critical in the pursuit of effective TIM-3 therapies for Alzheimer’s disease. The promising results observed in genetically modified mice reveal significant potential for human studies, allowing researchers to assess whether similar outcomes can be achieved in human populations. Testing human anti-TIM-3 antibodies on mouse models that carry the human TIM-3 gene serves as a vital step forward in the drug development pipeline.
Understanding the mechanisms through which TIM-3 impacts cognitive function in mouse models is essential for predicting outcomes in humans. The transition from innovative laboratory experiments to potential clinical trials reflects the commitment to developing targeted Alzheimer’s therapies that aim to reverse cognitive decline, highlighting the need for further research to establish the efficacy and safety of TIM-3 modulation in human subjects.
Alzheimer’s Research and Community Collaboration
Collaborative research efforts, such as those conducted between Vijay Kuchroo’s lab and the Ann Romney Center for Neurological Disease, exemplify the importance of interdisciplinary partnerships in advancing Alzheimer’s therapies. By pooling expertise, resources, and insights, researchers can tackle complex challenges in neurodegeneration more effectively. These collaborations are essential in accelerating discovery and creating innovative solutions that accommodate the multifaceted nature of Alzheimer’s disease.
Community engagement and partnership in Alzheimer’s research are also integral to fostering broad support and accelerating research translation into clinical settings. This holistic approach not only enriches the research landscape but also ensures that findings derived from laboratory studies can be harmonized with patient care strategies, ultimately benefiting those living with the disease.
The Future of Alzheimer’s Therapy: Hope and Challenges
The future of Alzheimer’s therapy is undoubtedly filled with hope yet accompanied by challenges. Advances in understanding immune modulation and the therapeutic potential of TIM-3 signify a paradigm shift that may lead to groundbreaking treatments. However, the transition from promising laboratory results to successful clinical applications requires navigating regulatory hurdles and ensuring that therapies are both safe and effective for diverse patient populations.
As researchers continue to explore the intricate relationship between the immune system and Alzheimer’s pathology, the ongoing quest for effective therapies remains crucial. By leveraging insights from both cancer strategies and immunological advancements, the potential for transformative therapies that address the cognitive decline associated with Alzheimer’s disease is becoming increasingly viable. This landscape of potential change brings both anticipation and the responsibility of ensuring that solutions translate well into clinical practice.
Frequently Asked Questions
What is TIM-3 and how does it relate to Alzheimer’s treatment?
TIM-3, or T-cell Immunoglobulin and Mucin-domain containing-3, is an immune checkpoint molecule that has been linked to late-onset Alzheimer’s disease. Research indicates that deleting TIM-3 allows brain immune cells, known as microglia, to effectively clear amyloid plaques, a hallmark of Alzheimer’s disease, thereby improving memory function in affected models.
How do immune checkpoint molecules like TIM-3 affect microglia in Alzheimer’s patients?
In Alzheimer’s patients, TIM-3 inhibits the activity of microglia, preventing them from clearing harmful amyloid plaques. By expressing high levels of TIM-3, microglia enter a homeostatic state, stopping them from performing their critical function of clearing debris in the brain, which contributes to cognitive decline.
Can TIM-3 inhibitors provide memory improvement in Alzheimer’s therapy?
Studies suggest that TIM-3 inhibitors can enhance microglial activity, leading to the clearance of plaques in Alzheimer’s models. This intervention has demonstrated potential for cognitive improvements, as microglia are freed from TIM-3’s inhibitory effects, allowing them to restore memory function.
What are the implications of TIM-3 research for future Alzheimer’s therapies?
The successful targeting of TIM-3 presents an exciting avenue for Alzheimer’s therapy. Anti-TIM-3 antibodies or small molecules could be developed to block TIM-3’s inhibitory effects, enabling microglia to better handle amyloid plaque accumulation and potentially slowing the progression of Alzheimer’s disease.
What role do microglia play in Alzheimer’s disease and how is TIM-3 involved?
Microglia are the primary immune cells in the brain responsible for clearing plaques. In Alzheimer’s disease, TIM-3 overexpression in microglia prevents these cells from phagocytosing plaques, which may lead to increased plaque buildup and cognitive impairment. Research into TIM-3 is crucial for devising new treatments to enhance microglial function.
How might cancer treatment strategies influence Alzheimer’s therapy involving TIM-3?
Cancer treatments that target immune checkpoint molecules, such as TIM-3, might be repurposed for Alzheimer’s therapy. Since TIM-3 plays a similar inhibitory role in both cancer and neurodegenerative diseases, strategies developed for cancer treatment could potentially enhance microglial clearance of Alzheimer’s plaques.
What are the potential next steps in TIM-3 Alzheimer’s treatment research?
Future research involves testing human anti-TIM-3 antibodies in Alzheimer’s disease mouse models with the human TIM-3 gene. The goal is to observe whether these treatments can effectively halt plaque development and improve cognitive function in models of late-onset Alzheimer’s disease.
What were the key findings of studies investigating TIM-3 in Alzheimer’s treatment?
Recent studies highlight that deleting TIM-3 from microglia enables these immune cells to clear amyloid plaques from the brain, leading to improved cognitive behavior in mouse models. This suggests that TIM-3 is a promising target for developing therapies aimed at improving memory in Alzheimer’s patients.
| Key Point | Description |
|---|---|
| Study Overview | Research indicated that inhibiting TIM-3 may allow microglia to tackle Alzheimer’s plaques, improving memory in mouse models. |
| Significance of TIM-3 | TIM-3 is a checkpoint molecule that impedes immune cells from attacking Alzheimer’s plaques. |
| Microglial Function | Microglia are brain immune cells that normally clear debris; TIM-3 expression prevents them from doing so. |
| Potential Therapy | Targeting TIM-3 with antibodies may provide a new treatment pathway for Alzheimer’s. |
| Research Duration | The study took approximately five years to complete, involving multiple experiments. |
| Next Steps | Further testing of human anti-TIM-3 antibodies in Alzheimer’s mouse models is underway. |
Summary
TIM-3 Alzheimer’s treatment presents a promising avenue for addressing the debilitating effects of Alzheimer’s disease. By targeting the TIM-3 checkpoint molecule, researchers have demonstrated significant potential for restoring the functionality of microglia, which can clear amyloid plaques in the brain. This innovative approach may transform how we treat Alzheimer’s, offering hope for improved cognition and memory restoration in affected individuals.