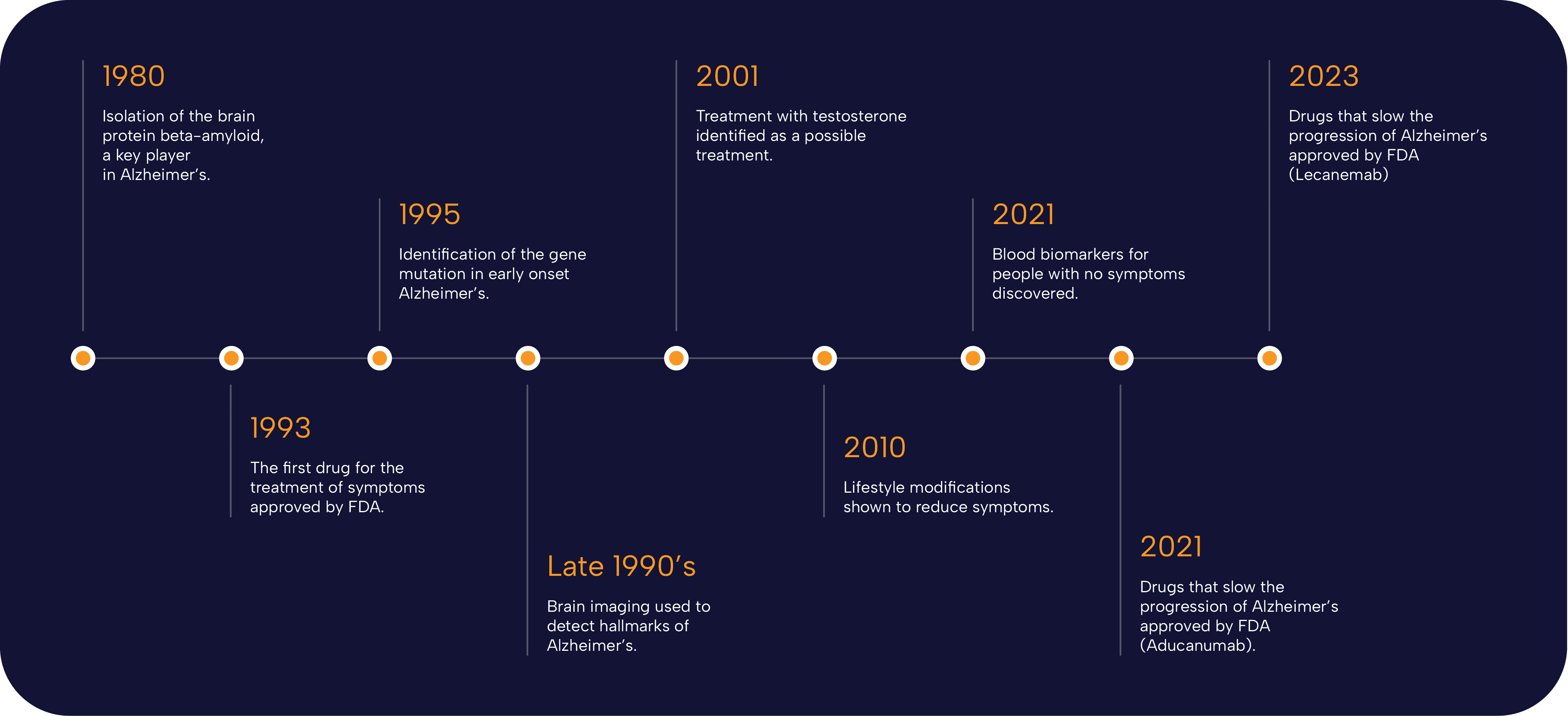
Recent advancements in Alzheimer’s research breakthroughs have revealed exciting insights into the role of microglial cells, which function as the brain’s immune system. These cells are pivotal in combating neurodegenerative diseases, actively monitoring and maintaining the health of neural pathways. A notable figure in this field is Beth Stevens, whose groundbreaking work has highlighted how maladaptive pruning by microglia may exacerbate conditions like Alzheimer’s. With the growing number of cases affecting millions of Americans, these treatment advances provide hope for earlier diagnosis and targeted therapies. As the research evolves, the discoveries made could reshape our understanding of brain health and open new avenues for combating Alzheimer’s disease.
Innovative developments in the field of Alzheimer’s disease research are bringing to light new possibilities for better treatments and early detection. Alternative terms like ‘cognitive decline’ and ‘memory loss disorders’ encompass the growing concerns associated with neurodegenerative conditions, and the strides being made in understanding cellular mechanisms are crucial. By studying the intricate actions of brain immune cells, researchers are revealing how these processes can either hinder or help brain function. Beth Stevens’ transformative research sheds light on how mismanagement of synaptic connections might lead to significant neurological disorders. As we stride into the future, these breakthroughs could revolutionize approaches to managing memory-related ailments and improve the quality of life for countless individuals.
Understanding Microglial Cells: The Brain’s Immune System
Microglial cells are fundamental components of the brain’s immune system, acting as the first line of defense against neurodegenerative diseases such as Alzheimer’s. These cells continuously monitor their environment, detecting signs of injury or disease within the brain. By clearing dead or damaged neurons and pruning synaptic connections, microglia maintain the health and functionality of neural circuits. However, recent research has revealed that when these cells become overactive or dysfunctional, they can inadvertently contribute to the progression of diseases like Alzheimer’s by incorrectly regulating synaptic pruning.
Beth Stevens, a leading neuroscientist in this field, highlights the significance of understanding microglial behavior in relation to neurodegenerative disorders. Her investigations into the immune responses of microglia have showcased how these cells can contribute to pathological processes in the brain. Through her work, she has set the groundwork for novel approaches to Alzheimer’s treatment advances, demonstrating that manipulating microglial activity might mitigate disease progression and improve outcomes for patients.
Alzheimer’s Research Breakthroughs: Paving the Way for New Treatments
Recent advancements in Alzheimer’s research have opened new avenues for understanding the complexities of the disease. Stevens alongside her team is at the forefront of these breakthroughs, having identified key mechanisms by which microglial cells might be harnessed for therapeutic purposes. By pinpointing the exact processes that lead to aberrant pruning of synapses, her work allows researchers to develop targeted interventions aimed at restoring normal microglial function and preventing cellular damage associated with Alzheimer’s.
The potential impact of these research breakthroughs is immense, particularly considering the alarming projections of an increasing Alzheimer’s population in the coming decades. As the demand for effective treatments grows, Stevens’ findings hold the promise of not just halting the progress of Alzheimer’s but also improving the quality of life for the millions affected. By focusing on the role of the brain’s immune system, researchers now have a pathway to create innovative Alzheimer’s treatment advances that leverage our understanding of neurodegenerative diseases at the cellular level.
The Role of Basic Science in Alzheimer’s Disease Research
Basic science research is critical to public health advancements, especially in complex conditions like Alzheimer’s disease. Beth Stevens emphasizes the importance of curiosity-driven research, which sets the foundation for discoveries that can eventually lead to practical treatments. Her work with microglial cells demonstrates how foundational studies of the brain’s immune system not only enhance our understanding but also lay the groundwork for clinical solutions. Such research often appears distant from real-world applications at its inception, yet it is precisely this groundwork that makes therapeutic advances possible.
The funding and support from organizations like the National Institutes of Health (NIH) have been pivotal in fostering this basic science. Without these essential resources, researchers would struggle to explore intricate mechanisms that underpin neurodegenerative diseases. By investing in basic research, the scientific community can drive forward initiatives that lead to effective diagnostics, early interventions, and ultimately better treatments for Alzheimer’s and other related disorders.
Neurodegenerative Diseases: A Growing Challenge for Society
As the global population ages, neurodegenerative diseases, particularly Alzheimer’s, present a growing challenge. Current estimates suggest that over 7 million Americans are living with Alzheimer’s, a number projected to double by 2050. The implications for healthcare systems and society at large are significant, with the Alzheimer’s Association estimating that the financial costs of care could skyrocket from $360 million to $1 trillion. This escalating burden underscores the urgent need for innovative research and treatment strategies.
The challenges posed by neurodegenerative diseases extend beyond numbers. They affect families and communities, reshaping lives and raising pressing questions about caregiving and support. Addressing these challenges requires not only scientific breakthroughs but also a commitment to understanding the broader implications of these diseases. Stevens’ work offers a beacon of hope, showing that with the right research, we can pave the way for advancements in care and treatment that will make a tangible difference in the battle against Alzheimer’s.
Beth Stevens: Inspiring the Next Generation of Neuroscientists
Beth Stevens’ journey as a neuroscientist illustrates the profound impact that individual curiosity and dedication can have on the field of Alzheimer’s research. Recognized as a MacArthur “genius,” her work has inspired many aspiring scientists to explore the complexities of the brain. By emphasizing the pivotal role of microglial cells in Alzheimer’s and other neurodegenerative diseases, she has motivated a fresh generation to engage with critical questions about brain health and disease mechanisms.
Her educational initiatives and public outreach also resonate with young researchers, showcasing how fundamental science can result in meaningful health outcomes. Stevens serves as a reminder that while the scientific path may not always be linear, curiosity and perseverance can lead to transformative discoveries that challenge the status quo and improve the lives of patients facing neurodegenerative diseases.
Exploring Disease Implications: From Mouse Models to Human Treatments
Many scientific breakthroughs originate from studies conducted on animal models, particularly mice. Stevens’ pursuit of understanding the visual system in these models reflects a broader trend in neuroscience where insights gained from basic research gradually translate into human applications. This process, while complex, allows researchers to test hypotheses that would otherwise be impossible in human subjects, ultimately contributing to the scientific foundation necessary for developing new therapies for diseases like Alzheimer’s.
Understanding how microglial cells operate in the context of neurodegenerative disease through mouse models offers crucial insights that inform potential human treatments. As studies progress, findings related to synaptic pruning and microglial behavior may yield strategies to modify or prevent the adverse effects of Alzheimer’s. This pathway underscores the intricate relationship between animal research and human healthcare developments, reinforcing the essential role of animal models in translating basic science into applicable treatments.
The Future of Alzheimer’s Treatments: Promising Directions
The landscape of Alzheimer’s treatments is evolving swiftly, propelled by breakthroughs in research and new technologies. As Stevens’ investigations continue to uncover the roles of microglial cells in neurodegeneration, the potential for novel treatment strategies becomes more apparent. The discovery of biomarkers related to microglial activity has provided a hopeful avenue for earlier detection of Alzheimer’s, allowing for interventions before significant cognitive decline occurs.
In addition to exploring the therapeutic manipulation of microglial cells, there is also a growing interest in integrating advancements in genomics and personalized medicine into the treatment of Alzheimer’s. The convergence of these fields promises to yield innovative solutions tailored to individual patients’ needs. With ongoing research and collaboration, the future holds the potential for more effective treatments that can significantly alter the course of Alzheimer’s disease.
The Impact of Federal Funding on Alzheimer’s Research
Federal funding plays an indispensable role in advancing Alzheimer’s research, facilitating the exploration of critical scientific questions. As highlighted by Stevens, much of her groundbreaking work emerged from substantial support from agencies like the National Institutes of Health (NIH). These investments not only allow researchers to pursue innovative ideas but also catalyze collaborative efforts across institutions and disciplines, enhancing the overall research landscape.
Investing in Alzheimer’s research through federal funding is essential for addressing the growing challenges posed by this disease. As the prevalence of Alzheimer’s increases, dedicated funding ensures that scientists can continue to explore the mechanisms of neurodegenerative diseases, investigate therapeutic options, and ultimately improve the quality of life for millions of individuals affected by Alzheimer’s and related disorders.
Community Engagement: Raising Awareness for Alzheimer’s Research
Community engagement plays a vital role in raising awareness about Alzheimer’s and mobilizing support for research efforts. Initiatives led by scientists like Beth Stevens often extend beyond the laboratory to involve the public in educational programs, workshops, and outreach activities. By informing community members about the importance of neuroscience research and its implications for Alzheimer’s treatment, scientists can foster a supportive environment conducive to research funding and collaboration.
Encouraging dialogue between researchers and community members not only elevates the profile of Alzheimer’s research but also fosters empathy and understanding for those affected by the disease. As individuals become more informed and invested in the fight against neurodegenerative diseases, it creates a ripple effect that can influence policy, enhance funding opportunities, and ultimately contribute to a more robust support network for ongoing research efforts.
Frequently Asked Questions
What are the latest breakthroughs in Alzheimer’s research involving microglial cells?
Recent breakthroughs in Alzheimer’s research highlight the role of microglial cells, which function as the brain’s immune system. Beth Stevens and her team have discovered that these cells are vital in pruning synapses during brain development. However, aberrant microglial activity can lead to neurodegenerative diseases like Alzheimer’s. This research is pivotal as it opens up new avenues for Alzheimer’s treatment advances, aiming to harness these immune responses to develop targeted therapies.
How do microglial cells contribute to Alzheimer’s treatment advances?
Microglial cells are essential in maintaining brain health by clearing damaged cells and modulating synapse pruning. Beth Stevens’ findings suggest that dysfunctional microglial activity may contribute to Alzheimer’s progression. By understanding these mechanisms, researchers can devise new Alzheimer’s treatment advances that aim to correct abnormal pruning behaviors, potentially altering disease trajectories for those affected.
Who is Beth Stevens and what is her contribution to Alzheimer’s research breakthroughs?
Beth Stevens is a prominent neuroscientist at Harvard Medical School, known for her groundbreaking research on microglial cells. Her work has transformed our understanding of neurodegenerative diseases, particularly Alzheimer’s. Stevens’ discoveries about how microglia interact with brain synapses provide a foundation for developing innovative treatments and identifying biomarkers for early detection of Alzheimer’s.
What impact does research on microglial cells have on the understanding of neurodegenerative diseases like Alzheimer’s?
Research on microglial cells profoundly impacts our understanding of neurodegenerative diseases, including Alzheimer’s. It reveals how these immune cells can both protect and, when functioning improperly, contribute to disease progression. Beth Stevens’ studies show that understanding microglial activity is crucial for developing strategies to mitigate Alzheimer’s effects and lead to potential treatment breakthroughs.
What role do biomarkers play in the advancements of Alzheimer’s research?
Biomarkers are critical in Alzheimer’s research as they allow for the early detection of the disease. Beth Stevens’ work on microglial cells has led to the identification of potential biomarkers that signify neurodegenerative processes. Early identification can lead to timely interventions, paving the way for treatment advances that could significantly alter patient outcomes.
How is basic science crucial for Alzheimer’s research breakthroughs?
Basic science is foundational for Alzheimer’s research breakthroughs because it lays the groundwork for understanding complex biological processes. Beth Stevens emphasizes that curiosity-driven research on microglial cells has been essential in uncovering the relationship between the brain’s immune responses and disease mechanisms. This foundational knowledge is critical for translating scientific discoveries into practical therapies for Alzheimer’s.
| Key Point | Details |
|---|---|
| Microglial Cells Role | Microglia act as the brain’s immune system, clearing out dead cells and pruning synapses. |
| Aberrant Pruning | Improper pruning by microglia contributes to Alzheimer’s and other neurodegenerative diseases. |
| Research Foundation | Beth Stevens’ research is heavily supported by federal funding, particularly the NIH. |
| Impact on Patients | Research has the potential to help the 7 million Americans currently living with Alzheimer’s. |
| Future of Alzheimer’s Treatment | Research may lead to new biomarkers for early detection and novel medicines for treatment. |
| Population Statistics | The number of Alzheimer’s cases is expected to double by 2050, raising care costs significantly. |
Summary
Alzheimer’s research breakthroughs are paving the way for innovative treatments and early detection methods. Under the leadership of Beth Stevens, significant advancements have been made in understanding how microglial cells function in the brain. Their role in clearing out damaged cells and improperly pruning synapses has been recognized as a contributing factor to Alzheimer’s disease. With ongoing research fueled by substantial federal funding, the hope to improve the lives of millions suffering from this condition becomes more attainable.